
Study on the effect of melamine on the growth of streptococcus thermophilus
Tech Blog Study on the effect of melamine on the growth of Streptococcus thermophilus Streptococcus thermophilus is a key lactic acid bacterium widely used in

Melamine powder is a highly versatile chemical compound with extensive applications across numerous industries.
In this article, we will explore what is melamine made of? Including formula of melamine, chemical structure of melamine, melamine production process and melamine chemical properties.
Appearance: White powder
CAS NO.: 108-78-1
Melting point: 354 ℃
Boiling point: 557.54 ℃
Flash point: 325.2 ℃
EINECS No.: 203-615-4
UN NO.: 3263

Melamine, also known by its chemical name 2,4,6-triamino-1,3,5-triazine, has the following chemical formula:
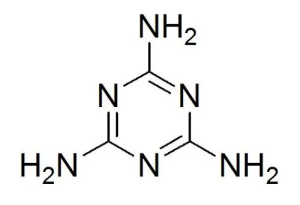
C3H6N6
Formula of melamine provides insights into melamine’s atomic composition, revealing its carbon (C), hydrogen (H), and nitrogen (N) atoms.

Tech Blog Study on the effect of melamine on the growth of Streptococcus thermophilus Streptococcus thermophilus is a key lactic acid bacterium widely used in

Tech Blog Melamine Modified Polyurethane Polyurethane (PU) is a versatile polymer celebrated for its excellent wear resistance, oil resistance, chemical stability, and strong adhesion to

Tech Blog Spectrophotometric Method for Melamine Detection The spectrophotometric method for melamine detection is based on the complexation reaction between melamine powder, formaldehyde, and carbonyl

JINGJIANG MELAMINE POWDER
© JINJIANG MELAMINE