
Study on the effect of melamine on the growth of streptococcus thermophilus
Tech Blog Study on the effect of melamine on the growth of Streptococcus thermophilus Streptococcus thermophilus is a key lactic acid bacterium widely used in

“Is urea corrosive?” This is a key question frequently raised in the fields of agriculture, transportation, and industry.
The answer is not simply ‘yes’ or ‘no’, but depends on its state of existence and environment. In short, dry urea has extremely low corrosiveness, but urea solution (especially when containing impurities) has significant corrosiveness.
This article will delve into the principle of urea corrosiveness, analyze its impact on different materials, and provide a practical set of prevention and management strategies.
Corrosion refers to the gradual deterioration of materials (usually metals) due to chemical reactions with the environment. Corrosive substances typically release ions that react with the metal surface, such as acids or bases, leading to rusting, pitting, or degradation.
To understand the corrosion behavior of urea, we must distinguish between two states:

Dry urea particles
In a completely dry state, solid urea particles themselves are chemically stable organic compounds with extremely low corrosiveness. It will not actively erode most metals or materials.
Urea solution (mixture of urea and water)
Pure urea solution is also non-corrosive, with a pH value close to 7 in fresh solution. It does not contain strong acids, strong bases, or oxidizing agents that corrode metals.
However, urea undergoes a hydrolysis reaction in water, slowly decomposing to produce ammonia and carbon dioxide. This process, especially in the presence of impurities, will form a corrosive electrolyte solution, thereby accelerating the electrochemical corrosion of metals.
Ammonia (NH3) dissolves in water to form ammonium hydroxide (NH4OH), a weak base with a high pH value (8-11). This alkaline solution can react with certain metals, leading to corrosion.
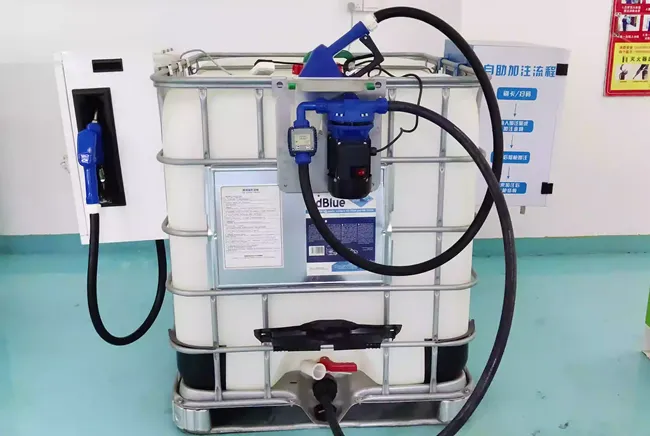
The corrosion effect of urea solution on different materials varies greatly. Understanding these differences is the foundation for choosing the right equipment and storage solutions.
Carbon Steel: Highly susceptible to corrosion. Carbon steel is a commonly used material for agricultural machinery and many storage tanks. Still, it is prone to corrosion in urea solutions with high ammonia content. Alkaline ammonium hydroxide reacts with iron in steel to form iron oxide and ammonium salts. Over time, this can cause pitting and thinning of the metal surface, which is why fertilizer spreaders must be thoroughly cleaned after use.
Aluminum: It is also susceptible to corrosion. Although aluminum has better corrosion resistance than carbon steel, it will corrode in high alkaline urea mixtures (pH>9). The hydroxide ions in the solution react with aluminum oxide (the protective layer on the surface of aluminum), decomposing it and further damaging the metal.
Risk scenario: Aluminum nozzle agricultural spray for application of concentrated urea-based foliar fertilizer.
Brass and Bronze: Copper and brass (an alloy of copper and zinc) are highly susceptible to corrosion in urea solution, especially in the presence of ammonia. Ammonia forms soluble complexes with copper ions, leading to rapid dissolution of metals, a process known as “ammonia erosion.”
Risk scenario: Pipeline system or industrial pipeline for transporting urea solution. Copper fittings can degrade, leading to leaks and contamination.
Stainless Steel: Has excellent corrosion resistance. 304 and 316L stainless steel are widely used in equipment for manufacturing and storing urea and diesel exhaust fluid (DEF/AdBlue). 316L stainless steel is a more ideal choice due to its higher resistance to pitting corrosion.
Plastic is the ideal material for storing and processing urea solution. Materials such as high-density polyethylene (HDPE), polypropylene (PP), and glass fiber reinforced plastic (FRP) have excellent chemical inertness to urea solutions and do not corrode, making them ideal choices for manufacturing urea storage tanks, pipelines, and pumps.
Agricultural applications: fertilizer spreaders, storage warehouses, irrigation systems (for water fertilizer integration), and any metal components in contact with urea fertilizers.
Automotive industry: storage tanks, pumping systems, and nozzles for diesel exhaust treatment fluid (DEF/AdBlue, 32.5% urea solution). Although these systems have been designed with corrosion resistance in mind, spills can still cause corrosion to the vehicle chassis and surrounding components.
Industrial production and transportation: equipment for urea production plants, truck carriages for transporting urea, railway freight cars, and port facilities.
In summary, dry urea itself is not considered a highly corrosive substance. However, under humid or warm conditions, its hydrolysis into ammonia significantly increases its corrosion potential, posing a serious threat to materials such as carbon steel, aluminum, and concrete. The key to success lies in managing the presence of ‘water’ and proactively preventing it by selecting suitable corrosion-resistant materials, implementing strict cleaning and maintenance procedures, and using protective coatings.
Is urea corrosive to the skin?
No, urea is a common ingredient in skincare products (moisturizers) that is gentle on the skin, but concentrated solutions may cause mild dryness.
Is urea corrosive to concrete?
Pure urea is not corrosive to concrete, but urea fertilizer will seep into the concrete over time, react with minerals to form salts, and cause slight cracks in poorly maintained structures.
Is urea more corrosive than salt?
No salt (sodium chloride) is highly corrosive to metals. At the same time, urea has a low risk of corrosion unless hydrolyzed into ammonia.

Tech Blog Study on the effect of melamine on the growth of Streptococcus thermophilus Streptococcus thermophilus is a key lactic acid bacterium widely used in

Tech Blog Melamine Modified Polyurethane Polyurethane (PU) is a versatile polymer celebrated for its excellent wear resistance, oil resistance, chemical stability, and strong adhesion to

Tech Blog Spectrophotometric Method for Melamine Detection The spectrophotometric method for melamine detection is based on the complexation reaction between melamine powder, formaldehyde, and carbonyl

JINGJIANG MELAMINE POWDER
© JINJIANG MELAMINE