Arc Resistance of Melamine Molding Compounds
Tech Blog Arc Resistance of Melamine Molding Compounds Melamine molding compounds are essential insulating materials for the electrical and instrumentation industries, widely used in mine


Melamine IUPAC name is “1,3,5-triazine-2,4,6-triamine”, and its chemical formula is C3H6N6.
This indicates that it is composed of three carbon (C) atoms, six hydrogen (H) atoms, and six nitrogen (N) atoms.
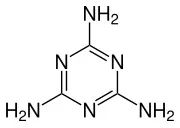
The carbon atoms in melamine form the backbone of its molecular structure.The carbon atoms are bonded in a specific arrangement in melamine powder that gives the molecule its unique shape and properties .
This structure is part of what allows melamine to have a relatively high melting point and good chemical stability.
The hydrogen atoms are attached to the carbon and nitrogen atoms. They play a role in determining the polarity of the melamine molecule.
Although the contribution of hydrogen atoms to the overall properties of melamine may seem less significant compared to carbon and nitrogen, they are essential for maintaining the molecular structure integrity.
The high proportion of nitrogen atoms is a distinctive feature of melamine. The nitrogen atoms are responsible for many of melamine’s key properties.
For example, the nitrogen – rich nature of melamine makes it useful in applications where a high nitrogen content is beneficial, such as in some fertilizers (in the form of its derivatives) and in certain types of resins.
The combination of carbon – carbon, carbon – nitrogen, and nitrogen – hydrogen bonds contributes to the high stability of melamine. These bonds are relatively strong, allowing melamine to withstand high temperatures and resist chemical degradation under certain conditions.
This stability is what makes melamine suitable for use in high – heat environments, such as in some kitchenware applications.
The arrangement of the atoms and the nature of the bonds also affect melamine’s solubility.
Melamine is slightly soluble in water at room temperature (about 3.1g/L) but has different solubility profiles in other solvents depending on the nature of the solvent and the interaction between melamine’s atoms and the solvent molecules.
For example, it is soluble in formaldehyde, acetic acid, hot ethylene glycol, glycerin, and pyridine, while it is insoluble in acetone, ethers, and carbon tetrachloride.
The components of melamine, namely carbon, hydrogen, and nitrogen atoms, are characterized by a hexagonal ring arrangement in its molecular structure, which contributes to its stability.
Understanding the relationship between its chemical composition and properties can help better utilize melamine in various industries, while also recognizing its potential limitations and safety issues.

Tech Blog Arc Resistance of Melamine Molding Compounds Melamine molding compounds are essential insulating materials for the electrical and instrumentation industries, widely used in mine

Tech Blog Influential Factors on Melamine Moulding Compounds Melamine moulding compounds are widely used in electronics, household utensils, and automotive parts due to their excellent

Tech Blog How to Slow Down Melamine Reactor Temperature Difference Rise Rate Melamine production via high-pressure processes (3rd-5th generation) relies heavily on reactor long-cycle operation.

JINGJIANG MELAMINE POWDER
© JINJIANG MELAMINE